
Water or H2O is a chemical substance that forms various kinds of bonds and reactions with other substances. Bonds hold water in place as well to form its structure. On this basis, then, is water an element or a compound?
While it is commonly referred to as an element due to its widespread presence across the world and even in the human body, does it structurally adhere to the definition of an element too?
To figure this out in further detail, you can go through the following sections where you will not only get a brief idea about water as an element or compound but also the reasoning behind the same, alongside some properties that make water different.
Elements and Compounds
Elements and compounds are two kinds of substances that are different from each other in terms of their structures.
Generally speaking, an element is a common word that might sometimes be used to refer to the four main natural elements on earth that include earth, air, fire and water. However, from a scientific point of view, an element is actually a substance that has a singular atom within its structure.
This means that it is the simplest or most basic form of a substance and cannot be broken down any further.
A compound, on the other hand, is more complex as compared to an element. It has two or more atoms within its structure and is comprised of two or more elements. A bond tends to form between the atoms or elements, resulting in the formation of a compound.
You should note that when the two or more elements come together to form a compound, the resulting compound has properties and characteristics of its own that the two elements may not have individually.
This means that it is also possible to break a compound down into smaller elements by carrying out a chemical change or reaction on it.
Is Water an Element or Compound?
Based on what we have seen, it is easy to conclude that water is, in fact, a compound and not an element. Let’s take a look at some properties that make water a compound.
- Water has two elements within its structure—hydrogen and oxygen. The atoms of both these elements bond to form the water compound that has two parts hydrogen and one part oxygen, hence the formula H2O.
- The bond between hydrogen and oxygen is covalent in nature, which means that the hydrogen and oxygen atoms share their valence electrons to achieve stability.
- The properties of water are different from the individual properties of both hydrogen and oxygen.
- You can conduct chemical changes or reactions with water that can break down the compound into its elements again, although this kind of reversibility cannot take place physically.
- Water, like other compounds, can also react with other compounds to form new elements and compounds, bringing about a distinct chemical reaction with new end products.
Water is also sometimes referred to as a molecule due to the bond that forms between the atoms of the elements. While this might sound the same as a compound, molecules can also simply contain a single atom, often existing naturally in the world in different forms.
Why Is Water Not Considered an Element?
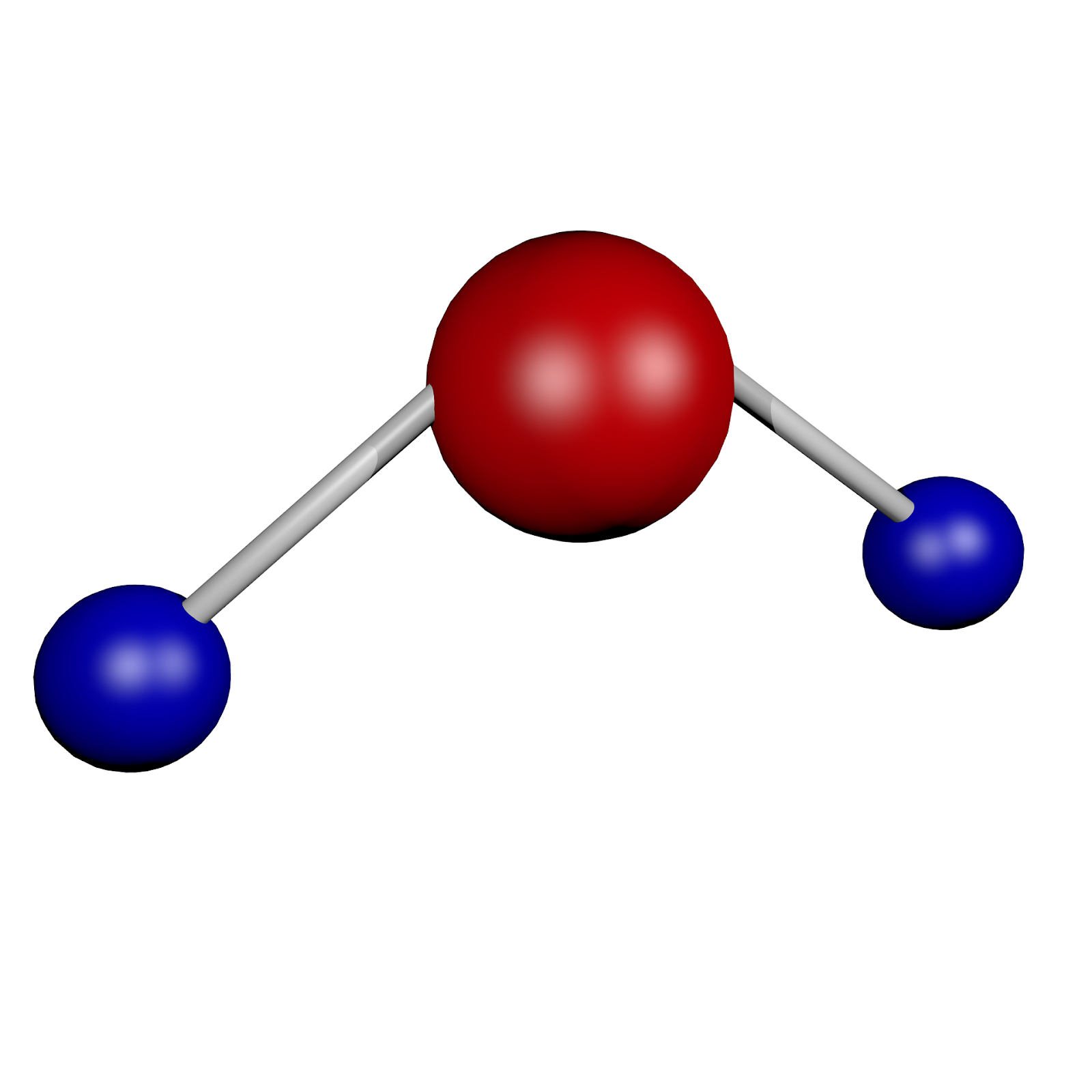
Water is a compound and not an element. What is it about water that does not earn it the classification of an element? Let’s find out.
- Elements have a single atom within their structure, thus disqualifying water as an element since it is formed by the bonds between the atoms of hydrogen and oxygen.
- Since water is made from two elements, the resulting substance is referred to as a compound due to the combination.
- You can break water down into smaller parts, something that is not possible with elements.
You might commonly hear people referring to water as an element, especially if they are speaking of it in the context of the earth, air and fire. While it is alright to refer to it as an element in such a case (since this is common parlance), it is incorrect to refer to it as an element in terms of chemistry and science.
Is Water a Mixture?
Water is not a mixture either. It might be easy to think of it as one since it combines the elements of hydrogen and oxygen. However, when a substance is a mixture, it involves the physical mixing and combination of substances. Thus, if you end up mixing water with another compound such as salt, then this is likely to give rise to a mixture.
Simply combining hydrogen and oxygen will result in the water molecule or compound. This is similar to most other compounds since they cannot form a mixture all by themselves.
What Makes Water Different?
There are several distinct properties of water that make it different from other similar compounds. It can be interesting to note these down to get a better understanding of why water is a compound and cannot possibly be an element.
For instance:
- When water freezes, it forms ice. Ice is lighter than water even though it takes on a solid state. You can test this by adding ice cubes to water or any other beverage.
- Water has the property of combining with numerous substances of all kinds with varying results.
- The boiling point of water is 100°C or 212°F, which tends to lower in the presence of atmospheric pressure.
- Water comprises a range of minerals that can prove to be quite helpful considering that it forms a large part of the Earth as well as the human body.
Concluding Words
Water is a compound made from the bonds that form between two hydrogen atoms and one oxygen atom. It is possible to break water down into these atoms or elements through chemical reactions, which is something that sets it apart from an element.
Water is also a distinct compound with properties of its own that set it apart from properties exhibited by various kinds of elements.